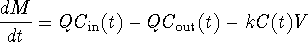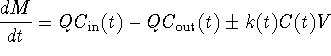
Now let's make an assumption about reaction kinetics. In many practical problems we deal with, if there is any type of reaction going on at all, we try to approximate it as a zero order or first order reaction. In this module, we will use that same approximation. We will not consider the case where contaminant is generated, so our k will always be non-negative. Thus our reaction rate k is given by some constant (zero if there is no reaction.) Thus we simplify by saying k(t) = k where k is a non-negative constant.
Thus our equation simplifies from
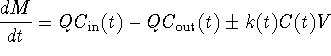
to the following: